Vis-U-Etch Cupric Chloride Etcher Controller
Oxford V.U.E., Inc.
11711 Clark St., Ste 108
Arcadia, CA 91006
USA
ph.(626) 256-6557
fax(626) 256-6567
www.oxfordvue.com
Vol. 5 Oxford V.U.E., Inc. Issue 2
News & VUEs
WHY ZERO NORMAL?
In describing the Vis-U-Etch™ 5 system and specifications, we routinely refer to the very important fact that our system operates at =<0.04 Normal free acid content or effectively, zero free acid. The question is, though, "Why is that so important?" The best way to describe this issue is with pictures.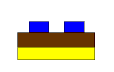
In the first picture, we see what the panel looks like as it leaves the developer on its way into the etcher. The blue represents the dry film, the brown is the copper foil and the yellow is the substrate.
In the second picture, etchant is sprayed from the nozzle, which starts to dissolve the exposed copper surface. At this point, it helps to understand exactly what is happening when you etch copper with cupric chloride. Cupric chloride (CuCl2) is simply one copper and two chlorine atoms. When CuCl2 comes in contact with copper (Cu), one of the chlorine (Cl) atoms from the CuCl2 molecule transfers to the Cu atom and you end up with two cuprous chloride (CuCl) molecules. Since there are no additional Cl atoms available for transfer, CuCl effectively becomes "dead" etchant. In order to continue etching, CuCl must find another Cl to become regenerated CuCl2 etchant.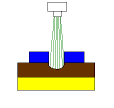
The third picture represents how the etching process occurs when there is excess hydrochloric acid (HCl) in the etchant. As you can see, the etchant puddle in the space etches sideways as well as down. This happens because CuCl is converted to CuCl2 in the space by having free HCl present. The Cl portion of HCl is your primary Cl source for regenerating CuCl into CuCl2. 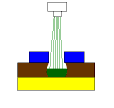 The higher the free HCl content, the more regeneration in the spaces can occur or the more sideways etching occurs. When trying to etch fine lines and spaces, this undercut makes the job more difficult. If the HCl content is allowed to be too high, fine lines and spaces become impossible. Increasing the free HCl content also has a tendency to increase the etch rate of Cu. Initially, this sounds like a good idea until you realize that, in order to reduce undercut, you have to expand the dry film width. Increasing the width of the dry film covers Cu that you want to etch actually slowing the etching process. With fine spaces, expanded dry film can make etching those spaces difficult or impossible.
The higher the free HCl content, the more regeneration in the spaces can occur or the more sideways etching occurs. When trying to etch fine lines and spaces, this undercut makes the job more difficult. If the HCl content is allowed to be too high, fine lines and spaces become impossible. Increasing the free HCl content also has a tendency to increase the etch rate of Cu. Initially, this sounds like a good idea until you realize that, in order to reduce undercut, you have to expand the dry film width. Increasing the width of the dry film covers Cu that you want to etch actually slowing the etching process. With fine spaces, expanded dry film can make etching those spaces difficult or impossible.
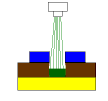 The final picture shows how the copper is etched at =<0.04N. Since there is no free HCl in the space, sideways etching is minimized. The reason is that etchant can only come from the spray nozzle which points down. When etchant is sprayed into the space, it hits the bottom first, converts from CuCl2 to CuCl and stops etching. The "dead" CuCl is only replaced by the CuCl2 coming from the spray nozzle, not regenerated by free HCl in the space.
The final picture shows how the copper is etched at =<0.04N. Since there is no free HCl in the space, sideways etching is minimized. The reason is that etchant can only come from the spray nozzle which points down. When etchant is sprayed into the space, it hits the bottom first, converts from CuCl2 to CuCl and stops etching. The "dead" CuCl is only replaced by the CuCl2 coming from the spray nozzle, not regenerated by free HCl in the space.
It is this principle that gives our "zero" free acid etchant controller superior etch factors (sidewall straightness) over any other system.
The following photos are actual cross-sections comparing the difference between the Vis-U-Etch™ 5 at zero Normal and ORP/Normality type systems at higher HCl Normality.
 The first cross-section photo shows the result of etching using the Vis-U-Etch™ 5 system at 0.04N on one-ounce copper.
The first cross-section photo shows the result of etching using the Vis-U-Etch™ 5 system at 0.04N on one-ounce copper.
The second cross-section photo shows an ORP/Normality controlled system using sodium chlorate based oxidizer operating at 1.0N on half-ounce copper.
The third cross-section photo shows an ORP/Normality controlled system using hydrogen peroxide operating at between 2.6N-3.2N on half-ounce copper.
 Aside from the obvious sidewall quality difference between the photos, it is important to realize that the Vis-U-Etch™ 5 cross-sections were performed using one-ounce copper while the other two were performed using half-ounce copper. Even with the inherent advantage the ORP/Normality systems had with thinner copper, the Vis-U-Etch™ 5 still handily out-performed both of them. It is also easy to see that, as you increase the amount of free HCl, the quality definitely goes down.
Aside from the obvious sidewall quality difference between the photos, it is important to realize that the Vis-U-Etch™ 5 cross-sections were performed using one-ounce copper while the other two were performed using half-ounce copper. Even with the inherent advantage the ORP/Normality systems had with thinner copper, the Vis-U-Etch™ 5 still handily out-performed both of them. It is also easy to see that, as you increase the amount of free HCl, the quality definitely goes down.
 To see the full parameters regarding these cross-section comparisons, please go to http://www.oxfordvue.com/TechInfo/cross-section_comparisons.html.
To see the full parameters regarding these cross-section comparisons, please go to http://www.oxfordvue.com/TechInfo/cross-section_comparisons.html.
It is also important to note that zero free acid is not all that contributes to higher etch factors. Etcher design parameters play a major role in this and were covered in our last newsletter.
If you did not receive the last newsletter or have lost it and would like another copy, just let us know and we will gladly send one to you.
ETCHER CIRCULATION
In a multi-chamber etcher, it is necessary to have a recirculating pump to enable proper blending of the regeneration chemistry with the cupric chloride in the etcher sump. The proper way to plumb the recirculation pump is to connect the feed to the pump from the front of the first etch chamber and the output from the pump to the rear of the final etch chamber. This ensures that the flow of etchant inside the etcher moves from last to first chamber, opposite the direction of the panels on the conveyor. On occasion, we have seen instances of the recirculation pump being plumbed the opposite direction and this can reduce the effective etch speed by as much as 10-15%. The reason is that most etching occurs in the first chamber(s) resulting in an increase in the amount of cuprous chloride in the first chamber(s), which does not etch. By having the flow of etchant moving the same direction as the panels, the cuprous chloride that forms follows the panels through the etcher, impeding the etch rate. By correctly having the flow of circulation in the etcher sump moving the opposite direction of the panels, you always have the fresh cupric chloride from the last chamber(s) moving towards the panels on the conveyor.
When connecting the Vis-U-Etch™ 5 to a multi-chamber etcher, it is best to sample from the first or second chamber for the Etch In and return the Etch Out approximately one-half to two-thirds the distance from the Etch In line back towards the last etch chamber.
DO NOT connect the Vis-U-Etch™ 5 to the recirculation pump! The flow of etchant through the recirculation pump would have to be drastically reduced in order to provide sufficient pressure to operate the Vis-U-Etch™ 5.
The minimum pump capacity and plumbing size is determined as follows. Select a pump and pipe size capable of moving the entire sump capacity of the etcher through the pump in five minutes or less (i.e., Fifty gallons per minute for a two hundred fifty-gallon total sump). Too little circulation can result in wider swings in the state of the etchant. Enough circulation ensures the most homogeneous etchant solution and consistent etched results.
WASTE TREATMENT
Over the past several years, there has been a tremendous increase in the number of PCB manufacturers switching to cupric chloride from ammonium chloride. The main reason for this change is that the industry as a whole is being asked to produce PCBs with smaller and smaller trace and space widths.
Ammonium chloride is great when you have larger traces with larger spaces and concern for a superior etch factor is not a requirement. As you get down to the finer lines, etch factor plays a major role in determining what is possible to etch and this is where cupric chloride shows its superiority.
This change to cupric chloride has been great for building better boards but there is a major item that needs to be addressed, proper handling of your spent (waste) etchant. When sending waste etchant to your recycler, it is important to be sure that it can be handled safely in transit and when it arrives at the waste treatment facility. I have had several conversations with customers of the Vis-U-Etch™ 5 and other controllers recently regarding etchant that was rejected for pickup or at the waste treatment facility because of chlorine gas problems.
In the USA, there are many companies that can handle waste etchant but most are local. The two largest North American recyclers are Phibro-Tech and Old Bridge Chemical Company. They can handle the collection of waste from anywhere in the USA and elsewhere but it is a real concern to them and others when it comes to chlorine gas caused by improperly handled control systems.
In order to ensure success in the removal of your waste etchant, it is important to maintain your etching equipment and controller properly at all times and, before calling for waste pick up, test the waste first.
Included with this newsletter are several helpful procedures you can use to help streamline your waste testing and collection with the least amount of problems. The testing procedures check for free acid normality (should be virtually zero with a Vis-U-Etch™ 5), free sodium chlorate and copper content. Also included is a copy of the test Phibro-Tech uses when arriving at your facility to check your waste for free chlorine.
If you are having problems with your spent etchant, the first thing to do is to make sure your controller is working properly and is calibrated correctly. For Vis-U-Etch™ 5 owners, checking the operation of the machine is easy and any questions about operation are welcomed and answered by contacting us at the telephone numbers listed in the footer of this newsletter or by e-mail at pculpovich@oxfordvue.com
Another good idea for your waste etchant is to mix any clean, pure scrap copper in with the waste. This will accomplish two things. First, it is a very convenient way to get rid of scrap copper without adding volume to your spent etchant or having to deal with a separate waste stream. Second, if there is any free chlorine in the waste, the copper will consume it and correct the free chlorine problem. If there is no free chlorine in your waste, adding the extra copper won’t hurt either because it just guarantees that you have "dead" etchant which makes the etchant safer and the recyclers happy.
The amount of copper needed to neutralize your waste will vary depending on the size of your waste etchant tank and the condition of the etchant. If you have a significant chlorine problem with your waste and don’t have enough scrap copper, you can purchase a copper product from the recycler to neutralize the waste before collection by the recycler. This may sound like an extra expense but it’s better to do this than to have your waste rejected for pickup and not be able to etch any more because your waste tank is full. You would still have to neutralize the waste etchant anyway. The extra expense and aggravation involved in neutralizing your waste certainly provides a great incentive to be sure that your equipment is functioning properly to keep your costs to a minimum.
There is also currently a large surplus of spent cupric chloride in the marketplace. This has been caused by both the explosive growth in cupric chloride use and several waste treatment companies shutting down operations. In California, this has caused an inability for Phibro-Tech to handle all the incoming waste at the Santa Fe Springs facility. Since this problem started, I spoke with Marty Lieberman, National Sales Director for Phibro-Tech. Marty informed me that the situation is now much improved and they are expanding their capacity but it is important to keep in mind that the industry as a whole is still going to feel the pinch of reducing recycling capacity due to a loss of several recycling companies.
I spoke with Joel Bzura at Old Bridge Chemical Company in New Jersey. Joel said that they have not only recently expanded their operations to handle our spent etchant, but due to the unprecedented rise in spent cupric chloride etchant generation, they are looking to increase their capacity again. At this time, Old Bridge Chemical Company is still able to handle the incoming spent etchant.
The third person I spoke with is Fred Stewart of Micronutrients, Division Of Heritage Technologies. Fred informed me that they are still taking in spent etchant but that they are close to capacity like all the others. Fred also informed me that they are looking to expand as well.
I have provided in this newsletter a list of recycling companies including the three choices mentioned so far.
Fortunately, there are still many choices for spent etchant pickup. Although the current waste etchant situation is improving, it really is up to all of us to do our part to ensure that the entire process runs smoothly. If you have any questions regarding the operation of your Vis-U-Etch™ 5 or problems with handling your waste cupric chloride, don’t hesitate to give us a call.
WASTE ETCHANT RECYCLERS
Joel Bzura
Executive Vice President
Old Bridge Chemicals, Inc.
P.O. Box 194
Old Waterworks Road
Old Bridge, NJ 08857
Tel: (732) 727-2225
Fax: (732) 727-2653
Martin Bergstedt
U.S. Filter
2430 Rose Place
Roseville, MN 55113
Tel: (612) 638-1330
Fax: (612) 633-5074
Steve Ansley
Phibro-Tech
8851 Dice Rd.
Santa Fe Springs, CA 90607
Tel: (310) 698-8036
Fax: (310) 698-1921
Ken Selby
Phibro-Tech
10 Industry Dr.
Joliet, IL 60435
Tel: (815) 727-4465
Fax: (815) 727-7545
Fred Stewart
Micronutrients, Div. Of
Heritage Technologies, L.L.C
1550 Research Way
Indianapolis, IN 46231
Tel: (317) 486-5880
Fax: (317) 486-5888
21st Century
Fernley, NV
Tel: (800) 648-9931
The Gni Group, Inc.
Deer Park, TX
Tel: 281-930-0350
PCI – Pollution Control Industries
Tel: (219) 397-3951
Romic
Tel: (800) 766-4248
Safety – Kleen
Tel: (888) 217-7859
US Filter
Vernon, CA
Tel: (323) 277-1500
If you know of any recyclers that are not listed here, please let us know so that we can update our database to provide the most complete list available to our customers and friends. Also, if there are any inaccuracies with these listings or a change of status with any of these companies, please let us know.
NEWSLETTER CREDITS
We would like to thank the following people and organizations for their help in providing the information in this newsletter:
Why Zero Normal?
Great Western Chemical Company – Cross-section photos.
Waste Treatment and Recycler List
Joel Bzura – Old Bridge Chemical
Marty Lieberman – Phibro-Tech
Dave DiMargo – Phibro-Tech
Fred Stewart – Micronutrients
IPC – Association Connecting Electronics Industries
Copper And Acid Normality Tests
Great Western Chemical Company
Free Sodium Chlorate Test
Ted Stern – Circuit Research Corp.
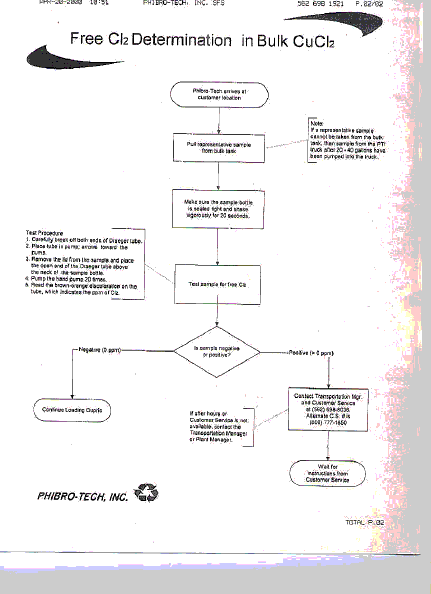
Free Cl2 Test For Bulk CuCl2 Etchant
Mark Alling – Phibro-Tech

TECHNICAL LITERATURE FINAL ETCHING
MODIFIED ANALYSIS FOR A CUPRIC CHLORIDE
ETCHING BATH UTILIZING RE/Gen CC-28
Control and Replenishment
It is necessary to periodically measure the copper concentration (as metal), hydrochloric acid normality, free sodium chlorate, and specific gravity to assure the feed rates of CC-28,
and hydrochloric acid are correct. Once the VIS-U-ETCHTM controller settings have been established, it is recommended that a sample be periodically tested by the laboratory to assure safe operation of the etchant.
1. SODIUM CHLORATE CONCENTRATION
Scope and Application
Chlorate is reduced with ferrous sulfate (added in excess) and the excess ferrous sulfate is back titrated with potassium dichromate in the presence of BDAS indicator. The color change of the redox reaction is green to purple. Please note the reaction stoichiometry is 6 mol potassium dichromate to every one mol of sodium chlorate.
Equipment Required
Buret, 50 ml
Erlenmeyer flask, 250 ml
Pipets, 5 and 25 ml
Reagents Required
BDAS indicator, (0.15% w/v (1.5 g/1000 ml) barium diphenyl amine sulfonate dissolved into
phosphoric acid, 75%)
Ferrous sulfate, standardized, 0.20 N
Phosphoric/sulfuric acid solution, (50% v/v phosphoric acid 75%, 25% v/v sulfuric acid, and
25% v/v DI water)
Potassium dichromate, 0.20 N
Procedure
1. Pipette a 10.0 ml sample of the cupric chloride solution into a 250 ml Erlenmeyer flask containing 50 ml of DI water. Add 25 ml of phosphoric/sulfuric acid solution.
3. Pipette 25.0 ml of ferrous sulfate, standardized, into the sample and heat to near boiling for three minutes. Allow to cool.
4. Add approximately 10-15 drops of BDAS indicator and titrate with potassium dichromate, standardized, from a green to a purple endpoint. Record the number of ml required as "S".
5. Ferrous sulfate, standardized is unstable! It is necessary to run a blank each day to account for changes in the ferrous sulfate concentration. To prepare a blank, pipette 25.0 ml of ferrous sulfate into a 250 ml Erlenmeyer flask containing 50 ml of DI water and 25 ml of phosphoric/sulfuric acid mixture. Add approximately 10-15 drops of BDAS indicator and titrate with potassium dichromate, standardized, from a green to purple endpoint. Record the number of required as "B".
Calculations
(B - S) x N x M x R = g/L sodium chlorate
V
Where B = ml titrant required for the "Blank"
S = ml titrant required for the "Sample"
N = N of potassium dichromate
M = M.W. of sodium chlorate (106.45)
R = Reaction stoichiometry (1:6 or 0.1667)
V = volume of sample in ml (10)
The typical range for free sodium chlorate in a working bath is 5-18 g/L. Adjustments to the sodium chlorate concentration are not necessary.
Statement of Proprietary Material: The information and descriptions contained herein are the property of CIRCUIT RESEARCH CORPORATION. Such information and descriptions may not be copied or reproduced by any means, or disseminated or distributed without the express prior written permission of CIRCUIT RESEARCH CORPORATION, 702 South 7th St., Delano, MN 55328.
OXFORD V.U.E., Inc.
PO Box 661896 Arcadia, CA 91066-1896
Tel. (626) 256-6557 - Fax (626) 256-6567 - www.oxfordvue.com
1. Pipette a 1.0 ml sample of cupric chloride etching solution into a 250 ml Erlenmeyer flask. For best accuracy, a "To Contain" (TC) pipette is recommended. After dispensing cupric solution from pipette wipe down the exterior of the pipette and rinse out the interior with DI water into the sample flask-total DI water addition should be approximately 50 ml.
2. Add 1-3 ml of 28% ammonium hydroxide to a deep blue color. Upon color change add a couple extra drops for confirmation of saturation.
3. Add 1-3 ml of concentrated acetic acid to a clear light blue solution. Upon color change add a couple extra drops for confirmation of saturation.
4. Add 4-5 grams potassium iodide to a dark almost transparent brown-again, excess is preferred.
5. Titrate with 0.10 normal sodium thiosulfate to a clear "water" color.
Calculations:
Copper by ounces per gallon = ml. sodium thiosulfate x 6.354
7.5
1. Put 10 ml etchant into 200 ml beaker.
2. Add 40 ml DI water. If solution becomes turbid (cloudy) stop normality is <= 0.005N.
3. If solution is clear blue titrate with 0.1N Sodium Hydroxide (NaOH). Add 0.1N NaOH until turbid (cloudy). Normality is milliliters of NaOH divided by 100.
Example: If 3 ml of NaOH is required, Normality is 0.03N
Note: Solution becomes turbid when hydroxide (OH) ions are available to bond with the CuCl2. This occurs at 2.9 pH. None of the common color indicators work accurately because the critical pH is 2.4 – 2.9 and a large amount of OH is absorbed by the CuCl2 without a change in pH.